PenTrac does more than merely automate routine tasks—it elevates your entire patient care workflow. PenTrac enhances patient care workflow by automating statistics and MQSA reports, the management of patient recalls and reminders, and letter generation, ultimately improving patient care and experience.
PenTrac’s AutoReader saves time, resources, and cost by pattern recognition technology to reduce the need for manual data input for tracking and statistical analysis. AutoReader automatically extracts information from reports even if it is generated through a 3rd party application. The user can leverage PenTrac while using a different preferred dictation system. AutoReader extracts exam information such as exam type, letter type, recall interval and BI-RADS. Information is stored for MQSA audit, tracking, statistics and unresolved case report generation.
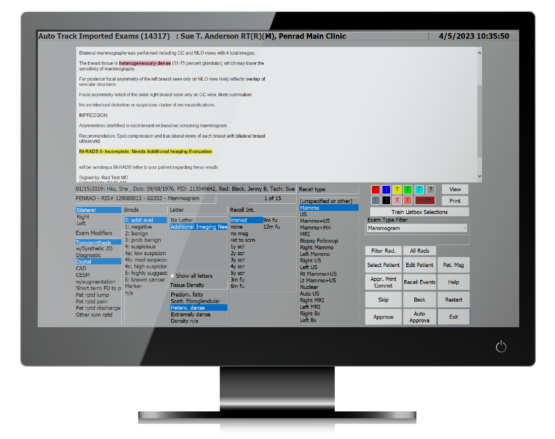

Experience seamless MQSA management with PenTrac. Benefit from advanced administrative reports tailored for program evaluation and data analytics. Automates the intricacies of patient tracking and enables customizable patient lay-letter generation and distribution in various languages.
Improve your patients’ experience by easily sending their results by email once their exam has been read. This also reduces the time and cost associated with mailing and handling expenses. This solution can even be customized to allow physicians to review communications prior to patient notification, and provide an audit trail for users to track messages, email, and phone results.
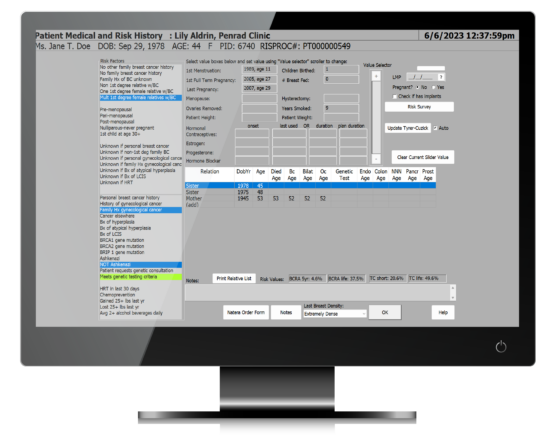
Allow patients to fill in required forms from the comfort of their home or provide them with mobile devices once they arrive in your facilities. Easily confirm previously entered information to speed up the process and make changes where needed.
© 2021 Intelerad Medical Systems Incorporated. All rights reserved.